 Port Credit Marine Survey & Yacht Delivery From Ontario, Canada Society of Accredited Marine Surveyors  American Boat & Yacht Council  Your Delivery & Survey Team  Our boat  Rudder stock  Chainplate  Chainplate  Keel bolt  Corroded propeller shaft  X-ray of a single pit in the propeller shaft  Sail drive  Outdrive  DC terminal  Fuel tank  Good drives  Keel bolt  Keel bolt  Keel bolt  |
Anodes, Zincs, Magnesium, Aluminum and Galvanic
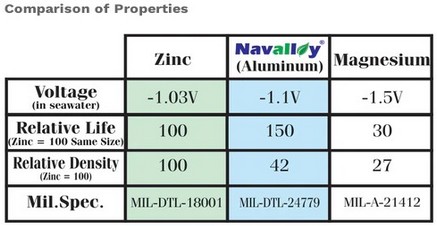
Corrosion on Boats Lets not call them "zincs",,,,,, they are "anodes" that may or may not be zinc. For the purpose of this article we are going to stick to strictly galvanic corrosion as it applies to your "anodes". Anode materials in order of decreasing galvanic activity ... MAGNESIUM anodes, generate -1.5 Volts, i.e. negative 1.5 Volts. Should be used only in fresh water as they are so active they don't last long enough in highly conductive salt or brackish water to be useful. ALUMINUM anodes, generate -1.1 Volts, i.e. negative 1.1 Volts The only ones that work and work well in salt, fresh and brackish water. and outlast the other two regardless of water due to its higher density.. ZINC anodes, generate - 1.03 Volts, i.e. negative 1.03 Volts and do not have high enough voltage potential to function in fresh water and mediocre performance (if at all in brackish water. Zinc also acquires an insulating,calcareous coating in fresh water which breaks the galvanic cell and renders them useless. This can happen within a few days of entering fresh water. PS. We are going to stick with fiberglass boats here, as steel, aluminum and wooden boats are different animals. |
||||
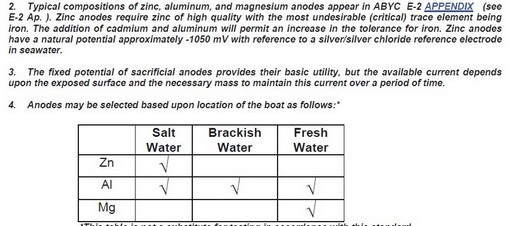 |
 |
||||
| Chart from ABYC E-2 Cathodic Protection shows
aluminum as the only anode material they
recommend for all waters |
Chart from Navalloy shows why voltage potential
of aluminum is recommended for all waters |
||||
| It is critical to use the correct anode material
for the type of water you cruise, salt, fresh
or brackish. Buying the correct anode is
not as simple as going to the chandlery and
asking for an aluminum alloy, magnesium or
zinc anode. "Zincs". have become
the "Kleenex" of the boating world,
a generic term many apply to all anodes.
"Anode" is the proper term. Zinc,
magnesium and aluminum alloy being the three
most common types of anodes. When you hear
the term "Cathodic Protection"
it refers to the protection offered in a
cathode-anode cell. On a recent visits to Ontario chandleries I found shelves of anodes mostly unidentified as to manufacturer, material or part number. Almost half of the anodes on the shelves were zinc which is a completely inappropriate material for use in the fresh water of the Great Lakes, some were aluminum and some were magnesium. Even if you find a part number on an anode and check the manufacturers website, they rarely relate that part number to a material. If your preferred chandlery displays their anodes like the photos below, tell them to clean up their act. |
|||||
Boxes of unidentified anodes are common in most chandleries Bins of unidentified anodes |
Three rare ianodes identified by material ! Material ??? |
||||
| NOT ALL ANODES ARE EQUAL If you buy cheap anodes they are likely coming from the same people that bulked up their powdered milk with Melamine (Google it). Or maybe the same people that caused a massive recall of Groco seacocks due to rapid corrosion with their questionable grasp of metallurgy (Google this one too). Buy nothing but US Mil.Spec anodes from name brand manufacturers. Saving $3 here will cost you in the long run. Some low quality anodes contain impurities that can cause the anode to consume itself through galvanic corrosion. |
|||||
| ANODE INSTALLATION A sound electrical connection between the anode and the metal it's protecting is absolutely critical and easily confirmed. An inexpensive multimeter set to Ohms (resistance) will instantly confirm if your anode has a good electrical connection to the metal it is supposed to protect, Touch one probe to the anode and one to the cathode (the metal being protected) and if you see "O.L" it means there is no electrical connection and therefore no protection. If you see more than 1.0 Ohms you have poor conductivity and the system is not working as well as it should. Standards call for a reading of less than 1.0ohm with a reading of 0.0 ohm being perfect. People often clean the exposed surface anodes with a wire brush but if a steel brush is used it can contaminate the anode with other metals that can render it useless. Cleaning the surface without cleaning the contact area (underneath) is also useless. Many people paint their stainless steel shafts with anti-fouling paint or even paint their anodes ! The paint does two negative things. 1st it prevents a good electrical contact of the anode if mounted on a painted surface and prevents the flow of electrons if painted over. 2nd if you paint your shaft you are also enhancing corrosion of the stainless steel by depriving it of the oxygen it needs to maintain it's anti-corrosion surface. The outdrive shown at right has it's anti-fouling paint applied properly, with a 1.5" unpainted border around it. I/O manufacturers will void your warranty if the paint touches the outdrive. Many anti-fouling paints contain some kind of metal content (cuprous oxide, carbon, titanium oxide etc.) If anti-fouling paint is in contact with the aluminum drive, the drive may become the anode and the entire bottom of the boat becomes the cathode. This turns everything below the waterline into a big battery and is a major contributor to drive corrosion. What is inexplicable is that so many so called "pro's" still paint right up to the drive.... Guess they never read the manufacturers warranty disclaimer. |
 O.L = "Open Line" in other words there is no electrical connection between this zinc anode and the shaft  Unpainted border around outdrive |
||||
| For an anode to work it requires a cathode
which by definition is a metal more
noble
on the galvanic scale than the anode. Are your anodes actually working ? While a multi-meter set to Ohms will tell you if there is enough conductivity for the anode to work. It cannot tell you how much current is flowing and whether it is enough or too much, for that you need a silver/silver chloride half cell (sometimes called a Corrosion Reference Electrode) which we should all have in our toolbox. I've provided the link so you can read up on that but It's really going beyond the scope of this article as my purpose here is just to get you using the correct anode material. This test is used by ABYC Certified Marine Corrosion techs to find stray current leaks and determine the level of galvanic current. |
|||||
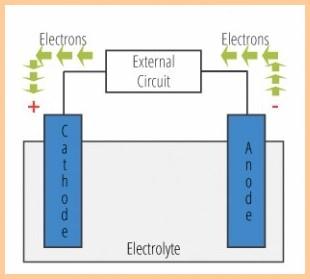
| NOW THE SCIENCE (the simplified version) Galvanic Corrosion - Electrolytic corrosion that occurs at the anode (zinc,magnesium,aluminum) of a galvanic cell. There are four elements required to form a galvanic cell, two metals of different potentials, a good electrical connection between those two metals and an electrolyte. Electricity must go around in a circle. i.e. It must go back to where it came from or it simply will not flow therefore a galvanic cell requires these four things. 1. An anode. 2. A cathode. 3. An electrolyte (the water). 4. A electric connection (path) betweeen the anode and the cathode. |
 Galvanic couple (cell) |
||||
| A galvanic couple (essentially a battery)
occurs when there is a voltage potential
difference between metals when these metals
are in electrical contact and submersed in an electrolyte. Note that "electrical contact" is underlined and the quality of this contact measured in Ohms is referred to as "continuity". The very definition of "galvanic couple" requires continuity between the metals involved. For an anode to do it's job there must be good continuity between the anode and the cathode (the metal it's supposed to protect) .Will the anode corrode without contact (continuity) ? ... it may as all metals do by many other means, it just may not be protecting the other metals as it does. Of two metals of different potential, say stainless steel and iron in a galvanic couple, the metal of lower nobility or lower on the galvanic scale (the iron) will give up electrons to the more noble metal and thereby corrode. Anodes are designed to be the lowest nobility
metals in the system and sacrifice (corrode)
themselves to protect the other more expensive
metals such as drive housings, propellers,
propeller shafts, shaft struts and rudders.
This action occurs faster in salt than fresh
water due to the conductivity of the electrolyte
involved although in rare cases, very polluted
fresh water can be even more conductive than salt water. Water temperature
also affects conductivity. |
|||||